Difficulty walking, blurred vision, slurred speech, slowed reaction times, impaired memory: Clearly, alcohol affects the brain. Some of these impairments are detectable after only one or two drinks and quickly resolve when drinking stops. On the other hand, a person who drinks heavily over a long period of time may have brain deficits that persist well after he or she achieves sobriety. Exactly how alcohol affects the brain and the likelihood of reversing the impact of heavy drinking on the brain remain hot topics in alcohol research today.
We do know that heavy drinking may have extensive and far–reaching effects on the brain, ranging from simple “slips†in memory to permanent and debilitating conditions that require lifetime custodial care. And even moderate drinking leads to short–term impairment, as shown by extensive research on the impact of drinking on driving.
A number of factors influence how and to what extent alcohol affects the brain (1), including
- how much and how often a person drinks;
- the age at which he or she first began drinking, and how long he or she has been drinking;
- the person’s age, level of education, gender, genetic background, and family history of alcoholism;
- whether he or she is at risk as a result of prenatal alcohol exposure; and
- his or her general health status.
This Alcohol Alert reviews some common disorders associated with alcohol–related brain damage and the people at greatest risk for impairment. It looks at traditional as well as emerging therapies for the treatment and prevention of alcohol–related disorders and includes a brief look at the high–tech tools that are helping scientists to better understand the effects of alcohol on the brain.
BLACKOUTS AND MEMORY LAPSES
Alcohol can produce detectable impairments in memory after only a few drinks and, as the amount of alcohol increases, so does the degree of impairment. Large quantities of alcohol, especially when consumed quickly and on an empty stomach, can produce a blackout, or an interval of time for which the intoxicated person cannot recall key details of events, or even entire events.
Blackouts are much more common among social drinkers than previously assumed and should be viewed as a potential consequence of acute intoxication regardless of age or whether the drinker is clinically dependent on alcohol (2). White and colleagues (3) surveyed 772 college undergraduates about their experiences with blackouts and asked, “Have you ever awoken after a night of drinking not able to remember things that you did or places that you went?†Of the students who had ever consumed alcohol, 51 percent reported blacking out at some point in their lives, and 40 percent reported experiencing a blackout in the year before the survey. Of those who reported drinking in the 2 weeks before the survey, 9.4 percent said they blacked out during that time. The students reported learning later that they had participated in a wide range of potentially dangerous events they could not remember, including vandalism, unprotected sex, and driving.
| Binge Drinking and Blackouts |
| • Drinkers who experience blackouts typically drink too much and too quickly, which causes their blood alcohol levels to rise very rapidly. College students may be at particular risk for experiencing a blackout, as an alarming number of college students engage in binge drinking. Binge drinking, for a typical adult, is defined as consuming five or more drinks in about 2 hours for men, or four or more drinks for women. |
Equal numbers of men and women reported experiencing blackouts, despite the fact that the men drank significantly more often and more heavily than the women. This outcome suggests that regardless of the amount of alcohol consumption, females—a group infrequently studied in the literature on blackouts—are at greater risk than males for experiencing blackouts. A woman’s tendency to black out more easily probably results from differences in how men and women metabolize alcohol. Females also may be more susceptible than males to milder forms of alcohol–induced memory impairments, even when men and women consume comparable amounts of alcohol (4).
ARE WOMEN MORE VULNERABLE TO ALCOHOL’S EFFECTS ON THE BRAIN?
Women are more vulnerable than men to many of the medical consequences of alcohol use. For example, alcoholic women develop cirrhosis (5), alcohol–induced damage of the heart muscle (i.e., cardiomyopathy) (6), and nerve damage (i.e., peripheral neuropathy) (7) after fewer years of heavy drinking than do alcoholic men. Studies comparing men and women’s sensitivity to alcohol–induced brain damage, however, have not been as conclusive.
Using imaging with computerized tomography, two studies (8,9) compared brain shrinkage, a common indicator of brain damage, in alcoholic men and women and reported that male and female alcoholics both showed significantly greater brain shrinkage than control subjects. Studies also showed that both men and women have similar learning and memory problems as a result of heavy drinking (10). The difference is that alcoholic women reported that they had been drinking excessively for only about half as long as the alcoholic men in these studies. This indicates that women’s brains, like their other organs, are more vulnerable to alcohol–induced damage than men’s (11).
Yet other studies have not shown such definitive findings. In fact, two reports appearing side by side in the American Journal of Psychiatrycontradicted each other on the question of gender–related vulnerability to brain shrinkage in alcoholism (12,13). Clearly, more research is needed on this topic, especially because alcoholic women have received less research attention than alcoholic men despite good evidence that women may be particularly vulnerable to alcohol’s effects on many key organ systems.
BRAIN DAMAGE FROM OTHER CAUSES
People who have been drinking large amounts of alcohol for long periods of time run the risk of developing serious and persistent changes in the brain. Damage may be a result of the direct effects of alcohol on the brain or may result indirectly, from a poor general health status or from severe liver disease.
For example, thiamine deficiency is a common occurrence in people with alcoholism and results from poor overall nutrition. Thiamine, also known as vitamin B1, is an essential nutrient required by all tissues, including the brain. Thiamine is found in foods such as meat and poultry; whole grain cereals; nuts; and dried beans, peas, and soybeans. Many foods in the United States commonly are fortified with thiamine, including breads and cereals. As a result, most people consume sufficient amounts of thiamine in their diets. The typical intake for most Americans is 2 mg/day; the Recommended Daily Allowance is 1.2 mg/day for men and 1.1 mg/day for women (14).
Wernicke–Korsakoff Syndrome
Up to 80 percent of alcoholics, however, have a deficiency in thiamine (15), and some of these people will go on to develop serious brain disorders such as Wernicke–Korsakoff syndrome (WKS) (16). WKS is a disease that consists of two separate syndromes, a short–lived and severe condition called Wernicke’s encephalopathy and a long–lasting and debilitating condition known as Korsakoff’s psychosis.
The symptoms of Wernicke’s encephalopathy include mental confusion, paralysis of the nerves that move the eyes (i.e., oculomotor disturbances), and difficulty with muscle coordination. For example, patients with Wernicke’s encephalopathy may be too confused to find their way out of a room or may not even be able to walk. Many Wernicke’s encephalopathy patients, however, do not exhibit all three of these signs and symptoms, and clinicians working with alcoholics must be aware that this disorder may be present even if the patient shows only one or two of them. In fact, studies performed after death indicate that many cases of thiamine deficiency–related encephalopathy may not be diagnosed in life because not all the “classic†signs and symptoms were present or recognized.
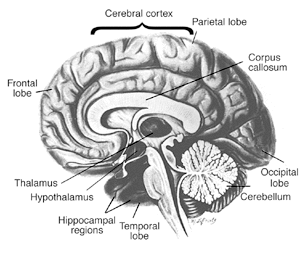
Approximately 80 to 90 percent of alcoholics with Wernicke’s encephalopathy also develop Korsakoff’s psychosis, a chronic and debilitating syndrome characterized by persistent learning and memory problems. Patients with Korsakoff’s psychosis are forgetful and quickly frustrated and have difficulty with walking and coordination (17). Although these patients have problems remembering old information (i.e., retrograde amnesia), it is their difficulty in “laying down†new information (i.e., anterograde amnesia) that is the most striking. For example, these patients can discuss in detail an event in their lives, but an hour later might not remember ever having the conversation.
Treatment
The cerebellum, an area of the brain responsible for coordinating movement and perhaps even some forms of learning, appears to be particularly sensitive to the effects of thiamine deficiency and is the region most frequently damaged in association with chronic alcohol consumption. Administering thiamine helps to improve brain function, especially in patients in the early stages of WKS. When damage to the brain is more severe, the course of care shifts from treatment to providing support to the patient and his or her family (18). Custodial care may be necessary for the 25 percent of patients who have permanent brain damage and significant loss of cognitive skills (19).
Scientists believe that a genetic variation could be one explanation for why only some alcoholics with thiamine deficiency go on to develop severe conditions such as WKS, but additional studies are necessary to clarify how genetic variants might cause some people to be more vulnerable to WKS than others.
LIVER DISEASE
Most people realize that heavy, long–term drinking can damage the liver, the organ chiefly responsible for breaking down alcohol into harmless byproducts and clearing it from the body. But people may not be aware that prolonged liver dysfunction, such as liver cirrhosis resulting from excessive alcohol consumption, can harm the brain, leading to a serious and potentially fatal brain disorder known as hepatic encephalopathy (20).
Hepatic encephalopathy can cause changes in sleep patterns, mood, and personality; psychiatric conditions such as anxiety and depression; severe cognitive effects such as shortened attention span; and problems with coordination such as a flapping or shaking of the hands (called asterixis). In the most serious cases, patients may slip into a coma (i.e., hepatic coma), which can be fatal.
New imaging techniques have enabled researchers to study specific brain regions in patients with alcoholic liver disease, giving them a better understanding of how hepatic encephalopathy develops. These studies have confirmed that at least two toxic substances, ammonia and manganese, have a role in the development of hepatic encephalopathy. Alcohol–damaged liver cells allow excess amounts of these harmful byproducts to enter the brain, thus harming brain cells.
Treatment
Physicians typically use the following strategies to prevent or treat the development of hepatic encephalopathy.
- Treatment that lowers blood ammonia concentrations, such as administering L–ornithine L–aspartate.
- Techniques such as liver–assist devices, or “artificial livers,†that clear the patients’ blood of harmful toxins. In initial studies, patients using these devices showed lower amounts of ammonia circulating in their blood, and their encephalopathy became less severe (21).
- Liver transplantation, an approach that is widely used in alcoholic cirrhotic patients with severe (i.e., end–stage) chronic liver failure. In general, implantation of a new liver results in significant improvements in cognitive function in these patients (22) and lowers their levels of ammonia and manganese (23).
ALCOHOL AND THE DEVELOPING BRAIN
Drinking during pregnancy can lead to a range of physical, learning, and behavioral effects in the developing brain, the most serious of which is a collection of symptoms known as fetal alcohol syndrome (FAS). Children with FAS may have distinct facial features (see illustration). FAS infants also are markedly smaller than average. Their brains may have less volume (i.e., microencephaly). And they may have fewer numbers of brain cells (i.e., neurons) or fewer neurons that are able to function correctly, leading to long–term problems in learning and behavior.
Fetal Alcohol Syndrome

Treatment
Scientists are investigating the use of complex motor training and medications to prevent or reverse the alcohol–related brain damage found in people prenatally exposed to alcohol (24). In a study using rats, Klintsova and colleagues (25) used an obstacle course to teach complex motor skills, and this skills training led to a re–organization in the adult rats’ brains (i.e., cerebellum), enabling them to overcome the effects of the prenatal alcohol exposure. These findings have important therapeutic implications, suggesting that complex rehabilitative motor training can improve motor performance of children, or even adults, with FAS.
Scientists also are looking at the possibility of developing medications that can help alleviate or prevent brain damage, such as that associated with FAS. Studies using animals have yielded encouraging results for treatments using antioxidant therapy and vitamin E. Other preventive therapies showing promise in animal studies include 1–octanol, which ironically is an alcohol itself. Treatment with l–octanol significantly reduced the severity of alcohol’s effects on developing mouse embryos (26). Two molecules associated with normal development (i.e., NAP and SAL) have been found to protect nerve cells against a variety of toxins in much the same way that octanol does (27). And a compound (MK–801) that blocks a key brain chemical associated with alcohol withdrawal (i.e., glutamate) also is being studied. MK–801 reversed a specific learning impairment that resulted from early postnatal alcohol exposure (28).
Though these compounds were effective in animals, the positive results cited here may or may not translate to humans. Not drinking during pregnancy is the best form of prevention; FAS remains the leading preventable birth defect in the United States today.
GROWING NEW BRAIN CELLS
For decades scientists believed that the number of nerve cells in the adult brain was fixed early in life. If brain damage occurred, then, the best way to treat it was by strengthening the existing neurons, as new ones could not be added. In the 1960s, however, researchers found that new neurons are indeed generated in adulthood—a process called neurogenesis (29). These new cells originate from stem cells, which are cells that can divide indefinitely, renew themselves, and give rise to a variety of cell types. The discovery of brain stem cells and adult neurogenesis provides a new way of approaching the problem of alcohol–related changes in the brain and may lead to a clearer understanding of how best to treat and cure alcoholism (30).
For example, studies with animals show that high doses of alcohol lead to a disruption in the growth of new brain cells; scientists believe it may be this lack of new growth that results in the long–term deficits found in key areas of the brain (such as hippocampal structure and function) (31,32). Understanding how alcohol interacts with brain stem cells and what happens to these cells in alcoholics is the first step in establishing whether the use of stem cell therapies is an option for treatment (33).
SUMMARY
Alcoholics are not all alike. They experience different degrees of impairment, and the disease has different origins for different people. Consequently, researchers have not found conclusive evidence that any one variable is solely responsible for the brain deficits found in alcoholics. Characterizing what makes some alcoholics vulnerable to brain damage whereas others are not remains the subject of active research (34).
The good news is that most alcoholics with cognitive impairment show at least some improvement in brain structure and functioning within a year of abstinence, though some people take much longer (35–37). Clinicians must consider a variety of treatment methods to help people stop drinking and to recover from alcohol–related brain impairments, and tailor these treatments to the individual patient.
Advanced technology will have an important role in developing these therapies. Clinicians can use brain–imaging techniques to monitor the course and success of treatment, because imaging can reveal structural, functional, and biochemical changes in living patients over time. Promising new medications also are in the early stages of development, as researchers strive to design therapies that can help prevent alcohol’s harmful effects and promote the growth of new brain cells to take the place of those that have been damaged by alcohol.
Source: https://pubs.niaaa.nih.gov/publications/aa63/aa63.htm
References
(1) Parsons, O.A. Alcohol abuse and alcoholism. In: Nixon, S.J., ed. Neuropsychology for Clinical Practice. Washington, DC: American Psychological Press, 1996. pp. 175–201. (2) White, A.M. What happened? Alcohol, memory blackouts, and the brain. Alcohol Research & Health 27(2):186–196, 2003. (3) White, A.M.; Jamieson–Drake, D.W.; and Swartzwelder, H.S. Prevalence and correlates of alcohol–induced blackouts among college students: Results of an e–mail survey. Journal of American College Health 51:117–131, 2002. (4) Mumenthaler, M.S.; Taylor, J.L.; O’Hara, R.; et al. Gender differences in moderate drinking effects. Alcohol Research & Health 23:55–64, 1999. (5) Loft, S.; Olesen, K.L.; and Dossing, M. Increased susceptibility to liver disease in relation to alcohol consumption in women. Scandinavian Journal of Gastroenterology 22: 1251–1256, 1987. (6) Fernandez– Sola, J.; Estruch, R.; Nicolas, J.M.; et al. Comparison of alcoholic cardiomyopathy in women versus men. American Journal of Cardiology 80:481–485, 1997. (7) Ammendola, A.; Gemini, D.; Iannacone, S.; et al. Gender and peripheral neuropathy in chronic alcoholism: A clinical–electroneurographic study. Alcohol and Alcoholism35:368–371, 2000. (8) Jacobson, R. The contributions of sex and drinking history to the CT brain scan changes in alcoholics. Psychological Medicine 16:547–559, 1986. (9) Mann, K.; Batra, A.; Gunther, A.; and Schroth, G. Do women develop alcoholic brain damage more readily than men? Alcoholism: Clinical and Experimental Research16(6):1052–1056, 1992. (10) Nixon, S.; Tivis, R.; and Parsons, O. Behavioral dysfunction and cognitive efficiency in male and female alcoholics. Alcoholism: Clinical and Experimental Research 19(3):577–581, 1995. (11) Hommer, D.W. Male and female sensitivity to alcohol–induced brain damage. Alcohol Research & Health 27(2):181–185, 2003. (12) Hommer, D.W.; Momenan, R.; Kaiser, E.; and Rawlings, R.R. Evidence for a gender–related effect of alcoholism on brain volumes. American Journal of Psychiatry 158:198–204, 2001. (13) Pfefferbaum, A.; Rosenbloom, M.; Deshmukh, A.; and Sullivan, E. Sex differences in the effects of alcohol on brain structure. American Journal of Psychiatry 158:188–197, 2001. (14) National Academy of Sciences. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. 1999. (15) Morgan, M.Y. Alcohol and nutrition. British Medical Bulletins 38:21–29, 1982. (16) Martin, P.R.; Singleton, C.K.; and Hiller–Sturmhöfel, S.H. The role of thiamine in alcoholic brain disease. Alcohol Research & Health 27(2):134–142, 2003. (17) Victor, M.; Davis, R.D.; and Collins, G.H. The Wernicke–Korsakoff Syndrome and Related Neurologic Disorders Due to Alcoholism and Malnutrition. Philadelphia: F.A. Davis, 1989. (18) Martin, P. “Wernicke–Korsakoff syndrome: Alcohol–related dementia.â€Â Family Caregiver Alliance Fact Sheet, 1998. (19) Cook, C. “The Wernicke–Korsakoff syndrome can be treated.â€Â The Medical Council on Alcohol, vol. 19, 2000. (20) Butterworth, R.F. Hepatic encephalopathy—A serious complication of alcoholic liver disease. Alcohol Research & Health 27(2):143–145, 2003. (21) Mitzner, S.R., and Williams, R. Albumin dialysis MARS 2003. Liver International 23(Suppl. 3):1–72, 2003. (22) Arria, A.M.; Tarter, R.E.; Starzl, T.E.; and Van Thiel, D.H. Improvement in cognitive functioning of alcoholics following orthotopic liver transplantation. Alcoholism: Clinical and Experimental Research 15(6):956–962, 1991. (23) Pujol, A.; Pujol, J.; Graus, F.; et al. Hyperintense globus pallidus on T1–weighted MRI in cirrhotic patients is associated with severity of liver failure. Neurology 43:65–69, 1993. (24) Chen, W–J.A.; Maier, S.E.; Parnell, S.E.; and West, J.E. Alcohol and the developing brain: Neuroanatomical studies. Alcohol Research & Health 27(2):174–180, 2003. (25) Klintsova, A.Y.; Scamra, C.; Hoffman, M.; et al. Therapeutic effects of complex motor training on motor performance deficits induced by neonatal binge–like alcohol exposure in rats: II. A quantitative stereological study of synaptic plasticity in female rat cerebellum. Brain Research 937:83–93, 2002. (26) Chen, S.Y.; Wilkemeyer, M.F.; Sulik, K.K.; and Charness, M.E. Octanol antagonism of ethanol teratogenesis. FASEB Journal 15:1649–1651, 2001. (27) Spong, C.Y.; Abebe, D.T.; Gozes, I.; et al. Prevention of fetal demise and growth restriction in a mouse model of fetal alcohol syndrome. Journal of Pharmacology and Experimental Therapeutics 297:774–779, 2001. (28) Thomas, J.D.; Fleming, S.L.; and Riley, E.P. Administration of low doses of MK–801 during ethanol withdrawal in the developing rat pup attenuates alcohol’s teratogenic effects. Alcoholism: Clinical and Experimental Research26(8):1307–1313, 2002. (29) Altman, J., and Das, G.D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. Journal of Comparative Neurology 124(3):319–335, 1965. (30) Crews, F.T., and Nixon, K. Alcohol, neural stem cells, and adult neurogenesis. Alcohol Research & Health 27(2): 197–204, 2003. (31) Nixon, K., and Crews, F.T. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. Journal of Neurochemistry 83(5):1087–1093, 2002. (32) Herrera, D.G.; Yague, A.G.; Johnsen–Soriano, S.; et al. Selective impairment of hippocampal neurogenesis by chronic alcoholism: Protective effects of an antioxidant. Proceedings of the National Academy of Science of the U.S.A. 100(13):7919–7924, 2003. (33) Crews, F.T.; Miller, M.W.; Ma, W.; et al. Neural stem cells and alcohol. Alcoholism: Clinical and Experimental Research 27(2):324–335, 2003. (34) Oscar–Berman, M., and Marinkovic, K. Alcoholism and the brain: An overview. Alcohol Research & Health 27(2):125–133, 2003. (35) Bates, M.E.; Bowden, S.C.; and Barry, D. Neurocognitive impairment associated with alcohol use disorders: Implications for treatment. Experimental and Clinical Psychopharmacology 10(3):193–212, 2002. (36) Gansler, D.A.; Harris, G.J.; Oscar–Berman, M.; et al. Hypoperfusion of inferior frontal brain regions in abstinent alcoholics: A pilot SPECT study. Journal of Studies on Alcohol 61:32–37, 2000. (37) Sullivan, E.V.; Rosenbloom, M.J.; Lim, K.O.; and Pfefferbaum, A. Longitudinal changes in cognition, gait, and balance in abstinent and relapsed alcoholic men: Relationships to changes in brain structure. Neuropsychology 14:178–188, 2000. (38) Rosenbloom, M.; Sullivan, E.V.; and Pfefferbaum, A. Using magnetic resonance imaging and diffusion tensor imaging to assess brain damage in alcoholics. Alcohol Research & Health 27(2):146–152, 2003. (39) Kensinger, E.A.; Clarke, R.J.; and Corkin, S. What neural correlates underlie successful encoding and retrieval? A functional magnetic resonance imaging study using a divided attention paradigm. Journal of Neuroscience 23(6):2407–2415, 2003. (40) Wong, D.F.; Maini, A.; Rousset, O.G.; and BrasÃc , J.R. Positron emission tomography—A tool for identifying the effects of alcohol dependence on the brain. Alcohol Research & Health 27(2):161–173, 2003. (41) Porjesz, B., and Begleiter, H. Alcoholism and human electrophysiology. Alcohol Research & Health 27(2):153–160, 2003. (42) Porjesz, B., and Begleiter, H. Human brain electrophysiology and alcoholism. In: Tarter, R., and Van Thiel, D., eds. Alcohol and the Brain. New York: Plenum, 1985. pp. 139–182. (43) Begleiter, H.; Porjesz, B.; Bihari, B.; and Kissin, B. Event–related potentials in boys at risk for alcoholism. Science 225:1493–1496, 1984. (44) Polich, J.; Pollock, V.E.; and Bloom, F.E. Meta–analysis of P300 amplitude from males at risk for alcoholism. Psychological Bulletin 115:55–73, 1994.
![]() recent blog entry, I wrote about how some of the young people I treat in my practice are using cannabis as a means of avoiding the anxiety-provoking stressors of becoming an adult and how that avoidance is contributing to a delay in maturation.
recent blog entry, I wrote about how some of the young people I treat in my practice are using cannabis as a means of avoiding the anxiety-provoking stressors of becoming an adult and how that avoidance is contributing to a delay in maturation.![]() damaged our credibility with a historic message that “all drugs are bad for all people all the time,” young users may be more willing to hear a more nuanced message. Two – there are real risks for young people who use cannabis daily. If they haven’t tried the drug, try to explain the benefits of waiting. Each year that passes before drug initiation lessens the risk of developing psychosis. If they have used the drug, and are unwilling to stop, adopt a position of harm reduction: any reduction of use is better than more use. Discuss changing the relationship with the drug to use less of it and to use it less frequently.
damaged our credibility with a historic message that “all drugs are bad for all people all the time,” young users may be more willing to hear a more nuanced message. Two – there are real risks for young people who use cannabis daily. If they haven’t tried the drug, try to explain the benefits of waiting. Each year that passes before drug initiation lessens the risk of developing psychosis. If they have used the drug, and are unwilling to stop, adopt a position of harm reduction: any reduction of use is better than more use. Discuss changing the relationship with the drug to use less of it and to use it less frequently.